HCl(aq) + NaOH(aq)
gcsescience.com 31 gcsescience.com
A Titration using an Acid and an Alkali.
The exact
amount of acid needed to
neutralise an
alkali
can be found by titration.
This technique can be used to
make
pure crystals
of a soluble salt
(one that dissolves in water).
In the example below
an
acid and an alkali react to make sodium chloride.
hydrochloric
acid
+ sodium hydroxide ![]() sodium chloride
+ water.
sodium chloride
+ water.
HCl(aq)
+
NaOH(aq)
![]() NaCl(aq)
+ H2O(l)
NaCl(aq)
+ H2O(l)
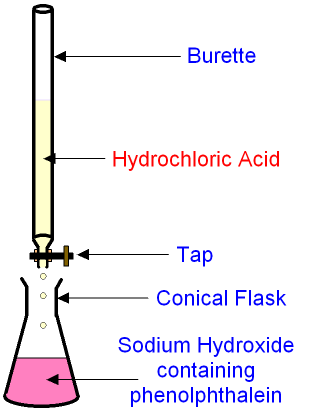
The burette is filled with hydrochloric acid.
A known quantity of alkali (say
50
cm3 sodium hydroxide)
is released from a pipette into the
conical flask.
The tap on the burette is turned open to allow
the acid to be added drop by
drop into the alkali.
The alkali contains
an
indicator (phenolphthalein)
which is pink in an alkali and colourless in an acid.
When enough acid has been added to neutralise
the alkali, the
indicator
changes from
pink to colourless. This is the end point of the
titration.
The titration can be repeated using the same amounts
of acid
and alkali but without the indicator.
Pure salt crystals which are free from indicator
can then be crystallised from the
neutral solution.
Using a pH meter.
You can use a pH meter to find the end point.
At neutralisation the pH is 7.
A pH meter allows you to make a pure salt sample faster
than an indicator because you do not have to repeat
the titration.
![]() Links
Acids
and Alkalis
Revision Questions
Links
Acids
and Alkalis
Revision Questions
![]()
gcsescience.com The Periodic Table Index Neutralisation Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.